Peptide Receptor Radionuclide Therapy (PRRT) for Gastric Neuroendocrine Tumors

Source: Shutterstock
Stomach (or gastric) cancer can be a scary diagnosis, but there are options available for treatment. Peptide receptor radionuclide therapy (PRRT) is a form of radiation therapy that may be recommended to patients with gastric neuroendocrine tumors. This article will explain how it works and its potential in cancer treatment. Being well-informed about PRRT can help you have valuable discussions with your cancer care team and prepare you for what to expect during your treatment journey.
What is a gastric neuroendocrine tumor?
Neuroendocrine tumors (NETs) are rare cancers that can occur anywhere in the body. Those that originate from neuroendocrine cells located in the mucosal lining of the stomach are called gastric NETs. These specialized cells produce hormones that control the release of gastric juices and how quickly food moves through the stomach.
What is peptide receptor radionuclide therapy (PRRT)?
PRRT is a type of internal radiation therapy that destroys cancer cells with a small but powerful radioactive dose. It is commonly used as a second- or third-line treatment for advanced gastroenteropancreatic NETs (GEP-NETs), particularly those that:
- are low-grade and well-differentiated (where the cancer cells look like normal cells),
- have somatostatin receptors overexpressed on the surface of cancer cells,
- have spread to other parts of the body or cannot be removed with surgery, and
- are no longer responding to first-line treatments, such as octreotide and lanreotide.
While PRRT is rarely able to cure your condition entirely, it can help to slow tumor growth, control the disease’s progression, relieve any symptoms and improve your quality of life.
How does PRRT work?
A distinctive feature of many NETs is that they have excessive amounts of proteins called somatostatin receptors on the surface of their cells. In normal circumstances, the hormone somatostatin binds to these receptors, triggering signals that regulate the production of various hormones.
PRRT makes use of radioactive drugs comprising three components: a radioactive substance called a radionuclide, a man-made form of the hormone somatostatin called a somatostatin analog (SSA) or peptide and a chelating agent that binds them together and stabilizes the drug. Together, this drug is called a radiopeptide or radiopharmaceutical.
Because of the SSA component, radiopharmaceuticals are able to bind to somatostatin receptors on the surface of tumor cells and deliver radiation to the NET directly. Subsequently, the radionuclide is internalized by the cancer cells, where it damages their DNA and destroys the cells.
In this way, PRRT combines the targeting properties of the SSA with the potent antitumor activity of radiation therapy. This type of treatment is also generally well-tolerated because it selectively attacks cancer cells while limiting damage to surrounding healthy cells.
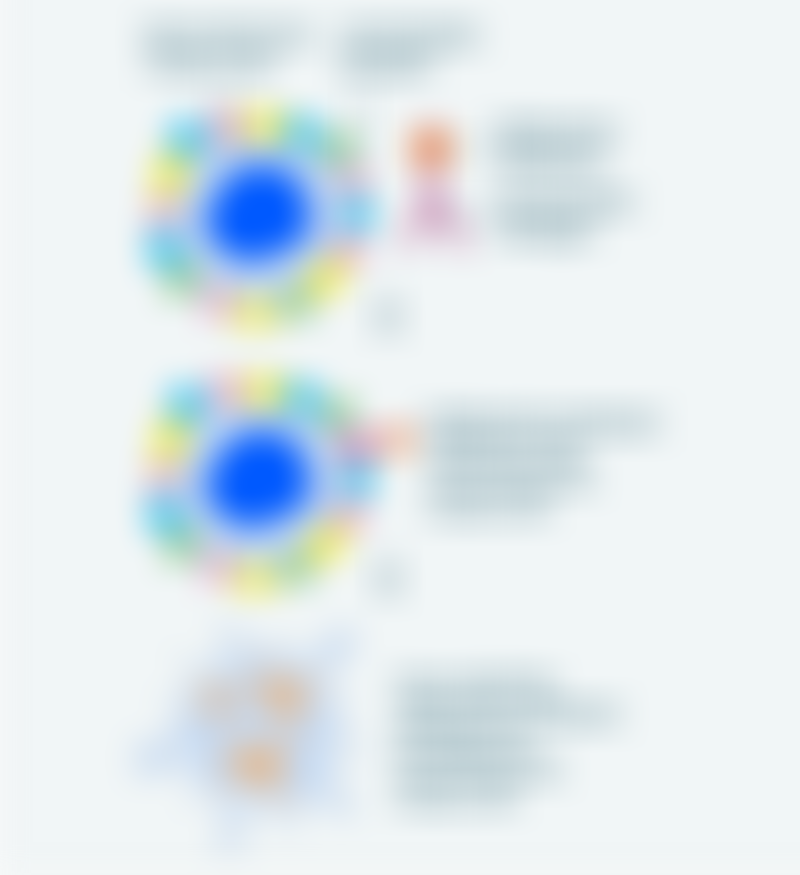
How PRRT works against NETs. Source: Cancer Research UK
How is PRRT administered?
PRRT is typically given over the course of four treatment sessions, with each session lasting approximately four to five hours. The sessions are usually spaced about eight weeks apart and the total duration for treatment can take up to 10 months.
Before each session, a thin tube called a cannula is inserted into your vein through which the radioactive drug will be injected into your bloodstream. You will also be given anti-nausea medication and an infusion of amino acids, the latter of which protects your kidneys from treatment-related effects. The administration of the radioactive drug only takes about 30 to 45 minutes.
PRRT for gastric NETs
The most widely used radiopeptide in PRRT is 177Lu-DOTATATE, also known by its commercial name Lutathera®. IIt consists of the SSA octreotate labeled with a radioactive element called lutetium-177. When injected into your bloodstream, the drug travels to the tumor(s) in the stomach and binds to the somatostatin receptors on the cancer cells. This delivers lutetium-177 directly to the gastric NET, where it emits radiation that destroys the cells from the inside.
PRRT has been used in the treatment of NETs for over two decades and there are many radiopharmaceuticals available in PRRT. However, 177Lu-DOTATATE is currently the only PRRT drug approved by the Food and Drug Administration (FDA) for treating GEP-NETs, including those originating from the stomach. This can be attributed to a number of reasons, which are the drug’s:
- high antitumor activity,
- higher affinity for somatostatin receptors expressed on the surface of cancerous neuroendocrine cells,
- lower toxicity levels, especially with regard to the kidneys, and
- overall ease of use.
This is especially true when compared to other radiopeptides, such as Y90-DOTATOC.
On top of its therapeutic effect, 177Lu-DOTATATE can be used for diagnostic radiography. This is because lutetium-177 emits two types of radiation: beta and gamma rays. While the former enables its use in the treatment of NETs, the latter allows this radiopeptide to be used in imaging scans.
What’s next for PRRT?
The field of PRRT continues to grow, with ongoing clinical trials evaluating the use of different radiopharmaceuticals, radionuclides and combination therapies. For instance, some are studying the use of radionuclides that emit alpha radiation, which can damage DNA to a greater extent. This could result in a higher antitumor effect while causing less damage to healthy tissue. Meanwhile, other trials are looking at combining PRRT with different treatments, such as chemotherapy, to enhance the cancer-killing effect on NETs. This is a promising and hopeful development for the future of cancer treatments.
If you have any questions regarding PRRT and whether it can help your treatment, please talk to your doctors and cancer care team. They may run some imaging scans or laboratory tests to find out if PRRT is suitable for you.