Immunotherapy for Colorectal Cancer

Source: Shutterstock.
Treatments for colorectal cancer can be roughly segregated into two categories: the conventional, tried-and-tested methods that have proven effective and novel treatments that bear much potential but do not always bear fruit.
A part of the latter is the most exciting up-and-coming treatment in recent years: immunotherapy. This article will explore what immunotherapy is, specifically for colorectal cancer, its effects and options for patients. This information may be useful for those with immunotherapy as part of their treatment plan as it can help with making more informed decisions and communicating more effectively with their cancer care team.
What is immunotherapy?
Immunotherapy can be broadly defined as using medicine to help a patient’s immune system better recognize and destroy cancer cells. The process by which this happens is, of course, a lot more complicated.
A normal, functioning immune system thrives on its ability to recognize foreign objects and threats in the body and remove them while also ensuring it doesn’t attack itself. Especially in the latter case, the immune system does so by using “checkpoints” consisting of proteins found on immune cells that are needed to start an immune response, either by activating or deactivating it. In the case of colorectal cancer, these checkpoints become altered so that the cancer cells avoid being attacked by T cells.
As such, immunotherapy can generally refer to all strategies that target the deregulated immune system that is responsible under normal conditions for maintaining immunosurveillance of human cells.
While immunotherapy may seem similar to chemotherapy, these treatments are distinctly different based on their primary function. Immunotherapy seeks to teach the immune system to recognize and fight the cancer, while chemotherapy is more direct in attacking cells that are undergoing differentiation and proliferation.
The efficacy of immunotherapy can be predicted by what is referred to as the ‘hotness’ or ‘coldness’ of a tumor. Hot tumors are characterized by tumor microenvironments with high levels of tumor-infiltrating lymphocytes (TILs), overexpression of programmed-death ligand 1 (PD-L1), genomic instability and pre-existing anti-tumor immune response. Cold tumors are the opposite; colorectal cancer tumors are not hot tumors, and so the efficacy of immunotherapy is normally limited.
However, immunotherapy has shown to be effective in one particular patient subgroup: those bearing tumors with DNA mismatch repair deficiency or high microsatellite instability (MSI-H).
What is microsatellite instability, and what does it have to do with immunotherapy?
The term ‘microsatellite’ refers to a tract of repetitive DNA that is found throughout the genome at thousands of locations. These repetitive sequences have a higher mutation rate compared to other DNA loci. Typically, when mutations result in erroneous insertions, deletions, or misincorporation of nucleotide bases, a system called DNA mismatch repair recognizes and repairs these errors. However, the errors may persist if the mismatch repair is not functioning correctly. This high instability within microsatellite sequences, or high microsatellite instability, is closely tied to Lynch syndrome, or occurs in sporadic tumors along with mutations of MAPK pathway members (Ras or Raf).
When it comes to recognizing cancer cells, the immune system’s T cells usually are not able to differentiate between cancer and normal cells, except in the case of high MSI colorectal cancer. When the mutation results in an overexpression of major histocompatibility complex (MHC) class I molecules, this attracts T cells into the tumor microenvironment.

T cell response to microsatellite-stable tumors and high MSI tumors.
Source: Journal of Gastroenterology
Tumors resulting from high MSI have been found to have an upregulation of immune checkpoint proteins, such as programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), compared to other types of cancer cells.
PD1 is a checkpoint protein that is found on T cells as well as other immune cells, while PD-L1 is a ligand found on the surface of healthy cells. When PD-L1 on a normal cell interacts with PD1 on a T cell by binding to it, it sends a signal that inhibits the T cell and prevents it from attacking the cell.
Some tumor cells are also able to express PD-L1 on the cell surface. From a T cell’s perspective, it cannot recognize a cancer cell that has been “disguised” to resemble a normal cell, i.e. when the PD-1 receptor on the T-cell successfully binds the PD-L1 protein on the tumor cell. This is the case with non-high MSI tumor cells, which do not respond well to immunotherapy treatments.
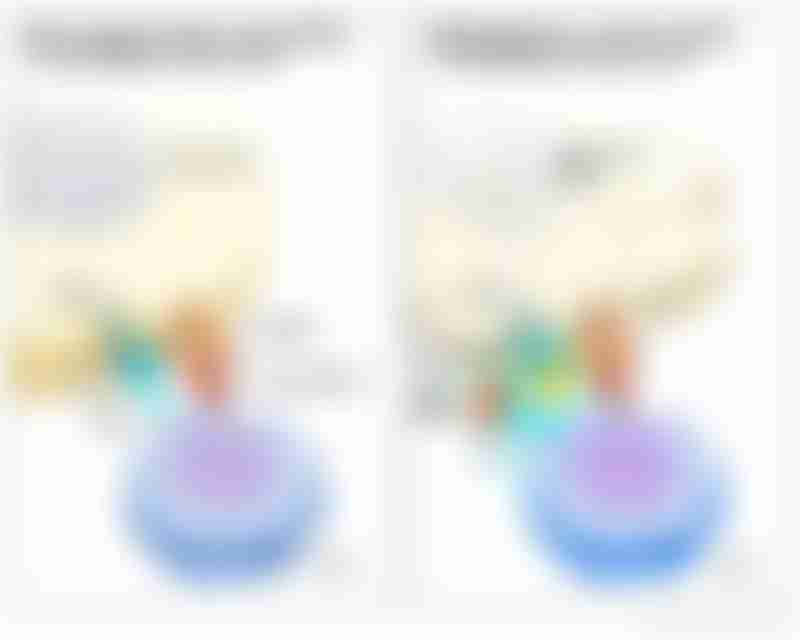
How immune checkpoint inhibitors work.
Source: National Cancer Institute
On the other hand, the interaction between the T-cell receptors with the MHC molecules, coupled with the addition of checkpoint protein inhibitors that block PD-1 and PD-L1, allows T-cells to recognize cancerous cells and activate programmed death, eliciting a more effective response in treatment.
Immunotherapy options
At present, there are four checkpoint inhibitors currently available as immunotherapy options. They are dostarlimab, nivolumab and pembrolizumab, which target the PD-1/PD-L1 checkpoint proteins, and ipilimumab, which targets the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathway.
In addition, monoclonal antibodies are also used in immunotherapy to inhibit angiogenesis — blood vessel growth by targeting either the target epidermal growth factor receptor (EGFR) or vascular endothelial growth factor (VEGF) pathways. They include bevacizumab, cetuximab, panitumumab and ramucirumab. As these specific monoclonal antibodies do not directly target the patient’s immune cells, these are considered targeted therapy drugs.
Side effects and shortcomings of immunotherapy
Like many other colorectal cancer treatments, immunotherapy is not without its side effects. Patients can expect to experience mild side effects, including
- Fatigue
- Cough
- Nausea
- Diarrhea
- Skin rash
- Loss of appetite
- Constipation
- Joint pain
- Itching
While less common, more severe side effects resembling allergic reactions are also possible. This will include symptoms such as fever, chills, breathing difficulties, rash and itchy skin, as well as dizziness.
In extreme cases, patients may have autoimmune reactions as a result of T cells targeting normal, healthy cells due to the introduction of checkpoint inhibitors. This can cause serious and life-threatening problems in other distant organs such as the lungs, liver and other parts of the body.
Under all circumstances, any new side effects must be reported to the treatment team immediately, as treatment may need to be halted while corticosteroids are administered to suppress the immune system and prevent further autoimmunity.
Besides the full spectrum of mild to severe side effects of immunotherapy, its current applications in treating colorectal cancer are also limited by its effectiveness. Colorectal cancer patients with metastatic cancer and high MSI make up just 3% of colorectal cancer cases, and while immunotherapy does effectively treat these advanced cancers, its effectiveness on other colorectal cancer types remains limited.
Although it may not be effective for all colorectal patients, for a small subset, immunotherapy has shown to be more specific and efficient than chemotherapy and other treatment methods, which often require combined usage. Despite its limitations, immunotherapy offers a glimmer of hope for some. Continued research and development in this field may lead to better cancer treatments in the future.