Current Developments in Colorectal Cancer Vaccines

Source: Shutterstock.
For many diseases, vaccines are deployed to protect the body against them, or to minimize the harmful effects that the disease may cause. In some cancers, vaccines also play similar preventive roles; the human papillomavirus (HPV) vaccine can prevent HPV infections and reduce the risk of cervical, vaginal and penile cancers. Preventive vaccines are usually administered before the onset of disease, or in the case of cancers, while they are in a premalignant state.
In the case of other cancers, vaccines may serve more therapeutic rather than preventive purposes. Nonetheless, both of these are considered a form of immunotherapy, as they involve enabling the immune system to recognize and eliminate cancer cells.
For colorectal cancer, the majority of vaccines under development are geared towards therapeutic measures, though at present, none of the vaccines have received FDA approval yet.
In this article, we take a deep dive into the colorectal cancer vaccines that are under development and get a closer look at what we can expect in the coming years.
BioNTech’s mRNA-based treatment vaccine
Now in the second phase of clinical trials, BioNTech’s colorectal cancer messenger RNA (mRNA) vaccine is one that aims to treat stage 2 and 3 colorectal cancer. In particular, the clinical trial is focused on the efficacy of the individualized cancer vaccine autogene cevumeran in high-risk stage 2 and 3 colorectal cancer patients who:
- Received surgery to remove colorectal tumors
- Have undergone and completed adjuvant chemotherapy
- Are circulating tumor DNA (ctDNA) positive following chemotherapy (ctDNA identified in blood samples)
At the moment, there is no standard treatment for this subset of colorectal cancer patients. Initial recruitment began at the National Institute for Health Research (NIHR) Clinical Research Facility (CRF) at Queen Elizabeth Hospital Birmingham. During the clinical trial, participants will receive 15 treatments over the course of one year, with their condition monitored and followed up on over at least 36 months.
How does the mRNA vaccine work?
mRNA vaccines typically encode tumor antigens, which are the markers that help the immune system differentiate cancer cells from normal, healthy cells. In this case, autogene cevumeran encodes up to 20 tumor-specific antigens (TSA) based on patient-specific cancer mutations.
TSAs, also known as neoantigens, are expressed exclusively in cancer cells and not in normal cells. Genetic mutations, such as those commonly associated with cancer, often lead to alterations of the resultant proteins they encode. These deformed proteins — the neoantigens — are recognized by the immune system as “non-self” and trigger an immune response. Unlike tumor-associated antigens (TAAs) that are expressed on both tumor and healthy cells at different concentrations, TSAs/neoantigens are highly specific, making them ideal for personalized immunotherapy treatments.
Clinical trial developments
Phase 1 of the trial showed promising results when autogene ceveruman was administered as monotherapy as well as in combination with atezolizumab, an anti-PD-L1 antibody, in patients with solid tumors. 161 patients were recruited for the trial, of which 29 patients were administered autogene ceveruman as monotherapy, while 132 received it in combination with atezolizumab.
Of the patients who received monotherapy, 4% showed an objective response — a measure of tumor burden in patients after receiving treatment. An objective response can be either:
- A complete response, i.e. a complete disappearance of tumor or lesion, or
- A partial response, i.e. the reduction in the sum of maximal tumor diameters by at least 30% or more
In addition, 40% of the monotherapy patients achieved stable disease, meaning that the cancer was not increasing or decreasing in extent or severity.
Treatment using combination therapy was also similarly successful. About 8% of the combination therapy patients showed an objective response, while 49% of them reached a state of stable disease.
At present, phase 2 of the trial is expected to run till 2027.
GRANITE: A vaccine study for metastatic, microsatellite stable colorectal cancer
Another vaccine that is currently in Phase 2/3, the GRANITE study is a neoantigen cancer vaccine program developed by Gritstone bio. Currently, the trial is focused on the maintenance activity with two patient-specific vaccines, GRT-C901 and GRT-R902. These vaccines are used in combination with checkpoint inhibitors such as pembrolizumab, nivolumab and ipilimumab, as well as fluoropyrimidine and bevacizumab.
Patients with the following characteristics were recruited:
- Confirmed metastatic colorectal cancer
- Received less than 30 days or were about to receive any of the following treatment combinations:
- FOLFOX with bevacizumab
- CAPEOX with bevacizumab
- FOLFOXIRI with bevacizumab
- CAPOXIRI with bevacizumab
- Microsatellite stable; patients with high microsatellite instability (MSI) were excluded
Trial participants first receive 24 weeks of induction therapy, where fluoropyrimidine, oxaliplatin or bevacizumab (with or without irinotecan) is administered. Thereafter, they will receive 6 administrations of GRT-C901/GRT-R902. In the first vaccine dose, ipilimumab is also co-administered.
Patients will also receive atezolizumab along with maintenance therapy consisting of fluoropyrimidine and bevacizumab.
What are GRT-C901 and GRT-R902?
GRT-C901 and GRT-R902 are viral-vector based vaccines. Viral vaccines utilize modified versions of a virus to deliver genetic material that encodes a specific antigen. GRT-C901 is an adenoviral vector developed from the chimpanzee adenovirus, and GRT-R902 is a fully synthetic Venezuelan equine encephalitis virus-based self-amplifying RNA (samRNA) vector.
GRT-C901 and GRT-R902 fall into the category of a prime-boost immunization strategy, where GRT-C901 is the neoantigen cancer vaccine prime, while GRT-R902 is the vaccine boost. The prime-boost strategy is an approach that can help develop greater levels of immunity as compared to singular vaccinations.
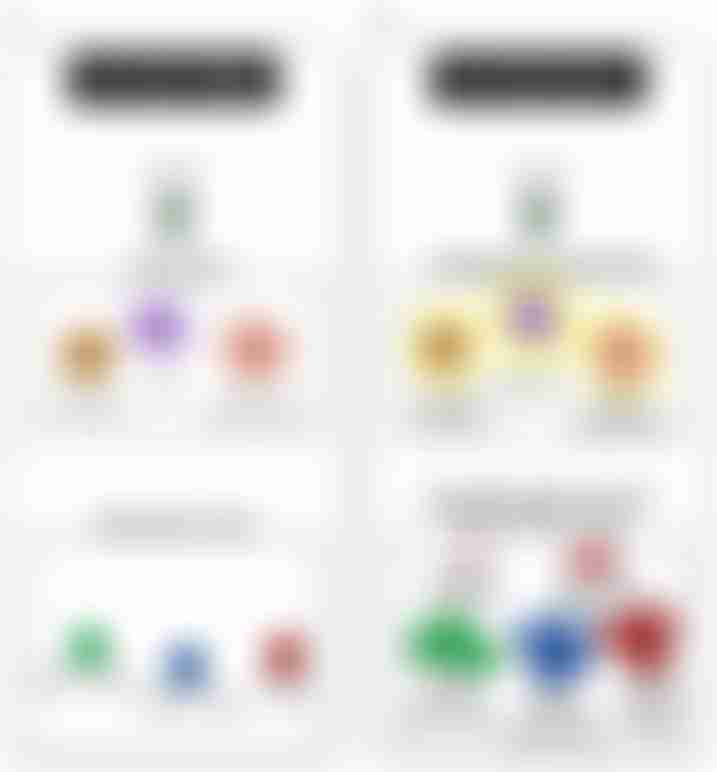
How a vaccine prime-boost strategy works.
Source: Palgen et. al.
The purpose of the vaccine prime is to help the immune system learn and recognize the immunogen. More specifically, the naïve B and T cells become activated. This process leads to the creation of long-lasting plasma cells, primary antibodies, and memory B and T cells. These memory cells have a heightened response when encountering the same threat again.
During a vaccine boost, the memory B and T cells are activated when they recognize the vaccine target. This subsequently leads to stimulation of the innate cells. The trained innate cells typically respond better during a boost compared to naïve cells, though the effectiveness depends on the vaccine type, how it’s administered and the time between doses.
Vaccine prime-boost strategies are either homologous or heterologous. A homologous strategy involves repeated administrations of the same vaccine for both the prime and boost, while a heterologous strategy uses different vectors or delivery systems that express the same antigenic inserts for the prime and boost.
GRT-C901 and GRT-R902 are an example of a heterologous strategy. Compared to a homologous strategy that is effective for augmenting humoral immune responses mediated by antibodies, a heterologous strategy is more effective at boosting cell-mediated immunity, which relies on mature T cell response to antigens.
In the case of GRT-C901 and GRT-R902, the viral vectors elicit immune responses to neoantigens that are specific to the patient’s cancer cells, thereby enabling the innate cells to act against and eliminate cancer cells without harming normal, healthy cells.
Clinical trial developments
During Phase 1/2 where 29 patients were recruited, an interim report provided promising news — the vaccine regime was deemed safe and well tolerated, and had no dose-limiting toxicities.
Patients with microsatellite-stable colorectal cancer also had improved overall survival based circulating tumor DNA (ctDNA) biomarker tests. Importantly, patients with longer overall survival had more frequent reductions in ctDNA compared to those with a median overall survival of 7.8 months.
- Of the 29 patients, three who had received treatment for less than six months showed an increase in ctDNA concentration.
- 75% of those treated for six months or more experienced a positive molecular response, such as a reduction of ctDNA by less than 50%.
- Two patients had completely cleared ctDNA levels during their treatment.
The present phase 2/3 study is expected to conclude in 2027.
While the two vaccines are currently still under clinical trial and are not expected to reach commercialization soon, the findings and development are nonetheless important in addressing the gaps in treatment for different colorectal cancer patient subtypes.